How Many Electrons Can a Single Orbital Hold
Quantum Numbers and Electron Configurations
Quantum Numbers
The Bohr model was a one-dimensional model that used one quantum number to describe the distribution of electrons in the atom. The only information that was of import was the size of the orbit, which was described by the northward quantum number. Schr�dinger's model immune the electron to occupy three-dimensional space. It therefore required three coordinates, or three quantum numbers, to describe the orbitals in which electrons tin be found.
The 3 coordinates that come from Schr�dinger's wave equations are the principal (n), angular (50), and magnetic (m) quantum numbers. These quantum numbers draw the size, shape, and orientation in space of the orbitals on an cantlet.
The master quantum number (n) describes the size of the orbital. Orbitals for which n = two are larger than those for which northward = i, for example. Because they have contrary electrical charges, electrons are attracted to the nucleus of the atom. Energy must therefore be captivated to excite an electron from an orbital in which the electron is shut to the nucleus (north = 1) into an orbital in which information technology is farther from the nucleus (n = 2). The principal quantum number therefore indirectly describes the energy of an orbital.
The angular quantum number (50) describes the shape of the orbital. Orbitals take shapes that are all-time described as spherical (l = 0), polar (l = 1), or cloverleaf (l = two). They tin even take on more circuitous shapes as the value of the angular breakthrough number becomes larger.

There is only one way in which a sphere (l = 0) tin be oriented in infinite. Orbitals that take polar (l = i) or cloverleaf (50 = 2) shapes, withal, can point in different directions. We therefore need a tertiary quantum number, known as the magnetic quantum number (k), to describe the orientation in space of a particular orbital. (Information technology is called the magnetic quantum number because the effect of different orientations of orbitals was start observed in the presence of a magnetic field.)
![]()
Rules Governing the Allowed Combinations of Quantum Numbers
- The three quantum numbers (n, 50, and chiliad) that describe an orbital are integers: 0, 1, ii, iii, and and so on.
- The primary quantum number (n) cannot be nada. The allowed values of n are therefore one, ii, 3, four, and then on.
- The angular quantum number (l) tin be whatever integer betwixt 0 and n - 1. If northward = 3, for example, l can be either 0, 1, or 2.
- The magnetic quantum number (1000) can exist any integer betwixt -l and +l. If l = 2, m can be either -2, -1, 0, +1, or +2.
![]()
Shells and Subshells of Orbitals
Orbitals that have the same value of the main breakthrough number form a shell. Orbitals within a shell are divided into subshells that have the aforementioned value of the angular quantum number. Chemists describe the beat and subshell in which an orbital belongs with a 2-character code such equally twop or fourf. The first character indicates the beat (n = 2 or n = four). The 2d grapheme identifies the subshell. By convention, the following lowercase letters are used to signal dissimilar subshells.
| s: | l = 0 | |
| p: | fifty = 1 | |
| d: | l = two | |
| f: | l = three |
Although there is no blueprint in the offset 4 messages (s, p, d, f), the letters progress alphabetically from that point (g, h, and and so on). Some of the allowed combinations of the n and fifty breakthrough numbers are shown in the effigy below.

The tertiary dominion limiting allowed combinations of the due north, fifty, and m quantum numbers has an important consequence. It forces the number of subshells in a shell to be equal to the primary quantum number for the shell. The n = 3 shell, for instance, contains three subshells: the 3s, iiip, and 3d orbitals.
![]()
Possible Combinations of Breakthrough Numbers
There is only one orbital in the n = ane crush because at that place is only one way in which a sphere tin be oriented in infinite. The only immune combination of breakthrough numbers for which n = 1 is the following.
There are iv orbitals in the n = 2 shell.
| 2 | i | -1 | | |||
| 2 | 1 | 0 | 2p | |||
| ii | 1 | one |
There is only one orbital in the iis subshell. Simply, there are three orbitals in the 2p subshell considering in that location are three directions in which a p orbital can point. One of these orbitals is oriented forth the X centrality, another forth the Y axis, and the third along the Z axis of a coordinate organization, as shown in the figure below. These orbitals are therefore known as the 2px , 2py , and 2pz orbitals.

At that place are 9 orbitals in the n = 3 shell.
There is one orbital in the 3south subshell and three orbitals in the iiip subshell. The n = three crush, however, also includes 3d orbitals.
The five different orientations of orbitals in the iiid subshell are shown in the figure below. 1 of these orbitals lies in the XY plane of an XYZ coordinate arrangement and is called the iiid xy orbital. The iiid xz and 3d yz orbitals take the same shape, simply they lie between the axes of the coordinate organization in the XZ and YZ planes. The quaternary orbital in this subshell lies along the X and Y axes and is called the iiid10 ii -y 2 orbital. Near of the infinite occupied by the fifth orbital lies along the Z centrality and this orbital is called the threedz ii orbital.
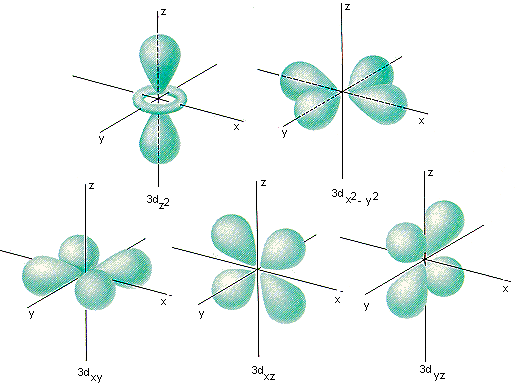
The number of orbitals in a shell is the square of the principal breakthrough number: onetwo = one, two2 = 4, 32 = 9. There is one orbital in an s subshell (l = 0), iii orbitals in a p subshell (l = ane), and five orbitals in a d subshell (l = 2). The number of orbitals in a subshell is therefore two(50) + ane.
Before we can use these orbitals we need to know the number of electrons that tin can occupy an orbital and how they can be distinguished from i another. Experimental evidence suggests that an orbital can agree no more 2 electrons.
To distinguish between the two electrons in an orbital, we need a fourth quantum number. This is called the spin quantum number (southward) because electrons acquit as if they were spinning in either a clockwise or counterclockwise manner. Ane of the electrons in an orbital is arbitrarily assigned an s breakthrough number of +1/2, the other is assigned an s quantum number of -1/2. Thus, it takes three quantum numbers to define an orbital but 4 quantum numbers to place one of the electrons that can occupy the orbital.
The immune combinations of n, 50, and 1000 quantum numbers for the first four shells are given in the table beneath. For each of these orbitals, there are two immune values of the spin quantum number, s.
![]()
Summary of Allowed Combinations of Breakthrough Numbers
| n | l | thousand | Subshell Notation | Number of Orbitals in the Subshell | Number of Electrons Needed to Fill up Subshell | Total Number of Electrons in Subshell | |||||
| ���������������������������������������������������������������� | |||||||||||
| 1 | 0 | 0 | 1s | 1 | 2 | 2 | |||||
| ���������������������������������������������������������������� | |||||||||||
| 2 | 0 | 0 | 2s | i | 2 | ||||||
| 2 | ane | i,0,-ane | 2p | 3 | 6 | eight | |||||
| ���������������������������������������������������������������� | |||||||||||
| 3 | 0 | 0 | 3s | one | 2 | ||||||
| 3 | i | i,0,-1 | 3p | 3 | 6 | ||||||
| 3 | 2 | two,i,0,-i,-2 | 3d | 5 | ten | 18 | |||||
| ���������������������������������������������������������������� | |||||||||||
| 4 | 0 | 0 | 4s | 1 | 2 | ||||||
| 4 | ane | 1,0,-1 | 4p | 3 | half dozen | ||||||
| 4 | 2 | 2,1,0,-1,-2 | 4d | 5 | 10 | ||||||
| 4 | iii | three,2,1,0,-ane,-ii,-iii | 4f | 7 | fourteen | 32 | |||||
![]()
The Relative Energies of Atomic Orbitals
Because of the force of allure betwixt objects of contrary accuse, the near of import factor influencing the energy of an orbital is its size and therefore the value of the principal quantum number, due north. For an atom that contains but one electron, there is no difference between the energies of the different subshells within a shell. The 3s, 3p, and 3d orbitals, for instance, accept the same energy in a hydrogen cantlet. The Bohr model, which specified the energies of orbits in terms of goose egg more than the distance betwixt the electron and the nucleus, therefore works for this atom.
The hydrogen atom is unusual, however. As soon as an atom contains more one electron, the unlike subshells no longer have the same energy. Within a given vanquish, the s orbitals e'er take the lowest energy. The free energy of the subshells gradually becomes larger as the value of the angular breakthrough number becomes larger.
Relative energies: s < p < d < f
As a result, ii factors control the energy of an orbital for near atoms: the size of the orbital and its shape, every bit shown in the effigy beneath.

A very unproblematic device can be constructed to approximate the relative energies of diminutive orbitals. The allowed combinations of the n and fifty breakthrough numbers are organized in a table, as shown in the figure below and arrows are fatigued at 45 caste angles pointing toward the lesser left corner of the table.

The order of increasing energy of the orbitals is then read off by post-obit these arrows, starting at the summit of the showtime line and then proceeding on to the 2nd, 3rd, fourth lines, and so on. This diagram predicts the following order of increasing energy for atomic orbitals.
anes < 2southward < 2p < 3south < iiip <fourdue south < iiid <4p < vsouth < 4d < 5p < 6s < 4f < vd < 6p < 7s < 5f < 6d < 7p < 8due south ...
![]()
Electron Configurations, the Aufbau Principle, Degenerate Orbitals, and Hund's Rule
The electron configuration of an atom describes the orbitals occupied past electrons on the atom. The basis of this prediction is a rule known as the aufbau principle, which assumes that electrons are added to an atom, 1 at a fourth dimension, starting with the everyman free energy orbital, until all of the electrons have been placed in an appropriate orbital.
A hydrogen atom (Z = ane) has merely one electron, which goes into the everyman energy orbital, the onesouthward orbital. This is indicated by writing a superscript "1" subsequently the symbol for the orbital.
H (Z = 1): 1s 1
The next element has two electrons and the second electron fills the 1due south orbital because there are only two possible values for the spin quantum number used to distinguish betwixt the electrons in an orbital.
He (Z = 2): isouthward 2
The 3rd electron goes into the next orbital in the energy diagram, the twos orbital.
Li (Z = 3): 1south 2 iis 1
The quaternary electron fills this orbital.
Exist (Z = 4): 1south 2 iis 2
After the is and 2s orbitals accept been filled, the next lowest free energy orbitals are the three iip orbitals. The 5th electron therefore goes into one of these orbitals.
B (Z = 5): 1due south 2 2due south 2 2p 1
When the time comes to add a sixth electron, the electron configuration is obvious.
C (Z = 6): ones 2 2south 2 twop 2
However, there are three orbitals in the 2p subshell. Does the 2nd electron get into the aforementioned orbital every bit the first, or does it get into one of the other orbitals in this subshell?
To answer this, we need to sympathise the concept of degenerate orbitals. By definition, orbitals are degenerate when they have the same energy. The free energy of an orbital depends on both its size and its shape because the electron spends more of its time further from the nucleus of the cantlet every bit the orbital becomes larger or the shape becomes more complex. In an isolated atom, yet, the energy of an orbital doesn't depend on the management in which it points in space. Orbitals that differ merely in their orientation in space, such every bit the 2px , twopy , and 2pz orbitals, are therefore degenerate.
Electrons fill degenerate orbitals according to rules kickoff stated past Friedrich Hund. Hund's rules tin can be summarized as follows.
- One electron is added to each of the degenerate orbitals in a subshell before two electrons are added to any orbital in the subshell.
- Electrons are added to a subshell with the aforementioned value of the spin quantum number until each orbital in the subshell has at least i electron.
When the time comes to place two electrons into the twop subshell we put one electron into each of two of these orbitals. (The choice between the 2p10 , 2py , and iipz orbitals is purely arbitrary.)
C (Z = vi): 1south ii 2south 2 2px ane 2py ane
The fact that both of the electrons in the 2p subshell have the aforementioned spin quantum number can be shown by representing an electron for which s = +1/ii with an
arrow pointing up and an electron for which due south = -1/2 with an pointer pointing down.
The electrons in the 2p orbitals on carbon can therefore be represented as follows.
![]()
When we go to N (Z = 7), nosotros have to put one electron into each of the three degenerate iip orbitals.
| N (Z = 7): | anes 2 2south 2 2p 3 | |
Because each orbital in this subshell now contains one electron, the adjacent electron added to the subshell must have the opposite spin quantum number, thereby filling one of the 2p orbitals.
| O (Z = viii): | 1south ii twosouthward 2 2p iv |  |
The ninth electron fills a 2d orbital in this subshell.
| F (Z = ix): | anes 2 2s ii twop five |  |
The tenth electron completes the 2p subshell.
| Ne (Z = 10): | 1s 2 iisouth 2 2p 6 |  |
At that place is something unusually stable about atoms, such as He and Ne, that have electron configurations with filled shells of orbitals. By convention, we therefore write abbreviated electron configurations in terms of the number of electrons beyond the previous element with a filled-trounce electron configuration. Electron configurations of the next two elements in the periodic table, for example, could exist written as follows.
Na (Z = 11): [Ne] iiisouth i
Mg (Z = 12): [Ne] threes 2
The aufbau process can be used to predict the electron configuration for an element. The bodily configuration used by the element has to be determined experimentally. The experimentally determined electron configurations for the elements in the showtime four rows of the periodic table are given in the table in the following section.
![]()
The Electron Configurations of the Elements
(1st, 2nd, 3rd, and 4th Row Elements)
| Atomic Number | Symbol | Electron Configuration | ||
| ���������������������������������������������������������������� | ||||
| one | H | 1s ane | ||
| ii | He | 1s 2 = [He] | ||
| 3 | Li | [He] 2southward 1 | ||
| 4 | Be | [He] twos 2 | ||
| v | B | [He] 2s 2 2p 1 | ||
| 6 | C | [He] 2s 2 iip 2 | ||
| 7 | N | [He] 2south 2 2p three | ||
| 8 | O | [He] 2s 2 2p 4 | ||
| 9 | F | [He] twos 2 twop v | ||
| 10 | Ne | [He] 2s 2 2p 6 = [Ne] | ||
| eleven | Na | [Ne] 3s 1 | ||
| 12 | Mg | [Ne] 3s 2 | ||
| 13 | Al | [Ne] 3s 2 threep 1 | ||
| 14 | Si | [Ne] 3due south two 3p ii | ||
| 15 | P | [Ne] 3s 2 3p 3 | ||
| sixteen | S | [Ne] 3s 2 threep 4 | ||
| 17 | Cl | [Ne] 3due south 2 3p 5 | ||
| 18 | Ar | [Ne] 3s ii 3p 6 = [Ar] | ||
| nineteen | K | [Ar] 4due south 1 | ||
| xx | Ca | [Ar] 4s ii | ||
| 21 | Sc | [Ar] fours ii iiid 1 | ||
| 22 | Ti | [Ar] ivs 2 3d two | ||
| 23 | 5 | [Ar] 4s two 3d 3 | ||
| 24 | Cr | [Ar] 4s 1 3d v | ||
| 25 | Mn | [Ar] 4s ii 3d 5 | ||
| 26 | Fe | [Ar] 4due south 2 threed 6 | ||
| 27 | Co | [Ar] ivdue south two 3d seven | ||
| 28 | Ni | [Ar] 4s ii iiid viii | ||
| 29 | Cu | [Ar] 4s 1 3d ten | ||
| xxx | Zn | [Ar] 4south 2 iiid 10 | ||
| 31 | Ga | [Ar] ivs 2 3d 10 4p i | ||
| 32 | Ge | [Ar] 4s 2 3d 10 ivp 2 | ||
| 33 | As | [Ar] 4s 2 3d 10 4p 3 | ||
| 34 | Se | [Ar] fours 2 3d x 4p 4 | ||
| 35 | Br | [Ar] 4s ii 3d 10 4p 5 | ||
| 36 | Kr | [Ar] 4south two iiid 10 4p 6 = [Kr] | ||
![]()
Exceptions to Predicted Electron Configurations
At that place are several patterns in the electron configurations listed in the tabular array in the previous section. One of the almost striking is the remarkable level of agreement between these configurations and the configurations we would predict. At that place are but two exceptions among the beginning twoscore elements: chromium and copper.
Strict adherence to the rules of the aufbau process would predict the following electron configurations for chromium and copper.
| predicted electron configurations: | Cr (Z = 24): [Ar] 4s 2 3d iv | |
| Cu (Z = 29): [Ar] 4s 2 3d 9 |
The experimentally adamant electron configurations for these elements are slightly different.
| bodily electron configurations: | Cr (Z = 24): [Ar] ivs 1 3d 5 | |
| Cu (Z = 29): [Ar] 4s one threed 10 |
In each case, i electron has been transferred from the 4southward orbital to a 3d orbital, even though the threed orbitals are supposed to be at a higher level than the ivs orbital.
One time nosotros get beyond diminutive number xl, the difference between the energies of adjacent orbitals is minor plenty that information technology becomes much easier to transfer an electron from one orbital to another. About of the exceptions to the electron configuration predicted from the aufbau diagram shown earlier therefore occur amid elements with atomic numbers larger than 40. Although information technology is tempting to focus attention on the scattering of elements that accept electron configurations that differ from those predicted with the aufbau diagram, the amazing thing is that this simple diagram works for so many elements.
![]()
Electron Configurations and the Periodic Table
When electron configuration data are arranged so that we can compare elements in i of the horizontal rows of the periodic table, we find that these rows typically represent to the filling of a shell of orbitals. The second row, for example, contains elements in which the orbitals in the northward = 2 shell are filled.
| Li (Z = 3): | [He] iis 1 | |
| Be (Z = 4): | [He] iis two | |
| B (Z = five): | [He] 2s ii 2p one | |
| C (Z = half-dozen): | [He] 2due south 2 2p 2 | |
| N (Z = 7): | [He] 2s ii iip 3 | |
| O (Z = viii): | [He] iis 2 iip 4 | |
| F (Z = 9): | [He] iisouth 2 iip 5 | |
| Ne (Z = 10): | [He] 2s two 2p 6 |
There is an obvious design inside the vertical columns, or groups, of the periodic table as well. The elements in a group have like configurations for their outermost electrons. This relationship can exist seen past looking at the electron configurations of elements in columns on either side of the periodic tabular array.
| Group IA | Group VIIA | |||||
| H | 1s ane | |||||
| Li | [He] 2s one | F | [He] 2s 2 2p 5 | |||
| Na | [Ne] 3s ane | Cl | [Ne] iiisouth two iiip 5 | |||
| K | [Ar] 4southward ane | Br | [Ar] 4s 2 3d x fourp five | |||
| Rb | [Kr] 5southward 1 | I | [Kr] fivesouth 2 4d 10 5p 5 | |||
| Cs | [Xe] 6s 1 | At | [Xe] sixsouth 2 4f 14 5d 10 6p 5 |
The effigy below shows the relationship between the periodic table and the orbitals being filled during the aufbau procedure. The 2 columns on the left side of the periodic table correspond to the filling of an south orbital. The next 10 columns include elements in which the five orbitals in a d subshell are filled. The vi columns on the right correspond the filling of the 3 orbitals in a p subshell. Finally, the 14 columns at the bottom of the table correspond to the filling of the seven orbitals in an f subshell.

![]()
Source: https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch6/quantum.html
0 Response to "How Many Electrons Can a Single Orbital Hold"
Post a Comment